Min.Order Quantity: 10 Ton
Port: Shanghai
Payment Terms: T/T
Methylethyl ketone is an organic compound with chemical formula of CH3COCH2CH3 and molecular weight of 72.11. It is a colorless transparent liquid with a smell similar to acetone. Volatile. It can be miscible with ethanol, ether, benzene, chloroform and oil. Soluble in 4 parts of water, but the solubility decreases when the temperature rises, and can form an azeotropic mixture with water. Low toxicity, half lethal dose (rat, oral) 3300mg/kg. Flammable, vapor can form explosive mixture with air. High concentrations of steam are narcotic.
Melting point: - 85.9 ℃
Density: 0.806g/cm3
Boiling point: 79.6 ℃
Saturated vapor pressure: 9.49kPa (20 ℃)
Combustion heat: 2441.8kJ/mol
Critical temperature: 260 ℃
Critical pressure: 4.40MPa
Logarithmic value of octanol/water partition coefficient: 0.29
Flash point: - 9 ℃ (CC)
Ignition temperature: 404 ℃
Upper explosion limit (V/V): 11.4%
Lower explosive limit (V/V): 1.7%
Appearance and properties: colorless liquid with acetone smell.
Solubility: soluble in water, ethanol, ether, and miscible in oils.
Methylethyl ketone is prone to various reactions due to its carbonyl group and the active hydrogen adjacent to the carbonyl group. Condensation occurs when heated with hydrochloric acid or sodium hydroxide to produce 3,4-dimethyl-3-hexene-2-one or 3-methyl-3-heptene-5-one. When exposed to sunlight for a long time, ethane, acetic acid, condensation products, etc. are generated. Diacetyl is formed when nitric acid is used for oxidation. Acetic acid is generated during oxidation with strong oxidants such as chromic acid. Butanone is relatively stable to heat, and it is thermally cracked to form ketene or methyl ketene at higher temperature. When condensed with aliphatic or aromatic aldehydes, high molecular weight ketones, cyclic compounds, ketals and resins are generated. For example, condensation with formaldehyde in the presence of sodium hydroxide will first produce 2-methyl-1-butanol-3-one, and then dehydration will produce methylisopropenyl ketone.
Resinification occurs when exposed to sunlight or ultraviolet light. Condensate with phenol to produce 2,2-bis (4-hydroxyphenyl) butane. React with aliphatic ester in the presence of alkaline catalyst to generate β- Diketone. In the presence of acid catalyst, it reacts with acid anhydride to form acylation reaction β- Diketone. It reacts with hydrogen cyanide to form cyanohydrin. It reacts with ammonia to produce ketopiperidine derivatives. Butanone α- The hydrogen atom is easily replaced by halogen to generate various halogenated ketones, such as 3-chloro-2-butanone. It reacts with 2,4-dinitrophenyl hydrazine to form yellow 2,4-dinitrophenyl hydrazone.
1. Used as cellulose acetate, acrylic resin, alkyd resin, paint, ink and other solvents, dye binder, lubricating oil dewaxing agent, curing agent, etc
2. Butanone is a reagent for measuring cadmium, copper and mercury, chromatographic analysis of reference materials and semiconductor photolithographic solvents
3. GB 2760-96 stipulates that edible spices are allowed. Butanone is mainly used to prepare cheese, coffee and banana essence. It can also be used as extraction solvent.
4. Butanone is mainly used as solvent for lubricating oil dewaxing, coating industry, various resin solvents, vegetable oil extraction process, refining process, etc. It has the advantages of strong solubility, lower volatility than acetone, and belongs to boiling point ketone solvent. Butanone is an intermediate for the preparation of pharmaceuticals, dyes, detergents, spices, antioxidants and some catalysts. Synthesis of anti-peeling agent methyl ethyl ketone pyridine, polymerization catalyst methyl ethyl ketone peroxide, anti-corrosion agent methyl pentanol, etc. It is used as a developer after lithography of integrated circuits in the electronic industry.
5. Butanone is the raw material for the preparation of acaricide Pyrimid.
6. Butanone is an organic synthetic raw material that can be used as a solvent. It is a dewaxing agent for lubricating oil in oil refining industry, and also used in medicine, coatings, dyes, detergents, perfumes and electronics industries. Liquid ink solvent. Cosmetics are used to make nail polish. As a low boiling solvent, it can reduce the viscosity of nail polish and dry quickly.
1. Dehydrogenation of sec-butanol
There are two methods of gas-phase and liquid-phase dehydrogenation. The gas-phase dehydrogenation uses zinc-copper alloy or zinc oxide as catalyst. The temperature is 400~500 ℃. The liquid-phase dehydrogenation uses Raney nickel or copper chromate as catalyst. The temperature is 150 ℃. The liquid-phase dehydrogenation reaction temperature and energy consumption are low. The yield is high. The catalyst life is long. The separation process is simple
2. Butane liquid-phase oxidation method
The main product of butane liquid-phase oxidation is acetic acid, and the byproduct butanone (about 16% of acetic acid production), the reaction temperature is 150~225 ℃, and the pressure is 4.0~8.0MPa. For example, the United Carbide Company of the United States produced 226000 tons of acetic acid in 1976 with this method, and 36000 tons of butanone were obtained. About 20% of butanone in the United States was produced with this method. As of 2011, the methods under research and development include butylene liquid-phase oxidation process and isobutyl benzene process
3. Butene liquid-phase oxidation method
This method is called the mutual Kerr method (Wacker method), which uses palladium chloride/copper chloride solution as the catalyst, and works at 90~120 ℃ and 1.0~2.0 MPa
CH2=CHCH2CH3[O2]→CH3COCH2CH3
The conversion rate of butene is about 95%, and the yield of butanone is about 88%. The reaction solution obtained is purified by distillation and other methods to obtain the finished product. The process is simple, but the equipment is severely corroded. Heavy metals are needed as catalysts. This method has not been applied to large-scale production
4. Isobutylbenzene method
Butene and benzene are alkylated to isobutyl benzene, isobutyl benzene is oxidized to isobutyl benzene hydroperoxide, and finally butanone and phenol are decomposed by acid
Benzene alkylation takes aluminum trichloride as catalyst, reaction temperature is 50~70 ℃, isobutyl benzene is obtained, isobutyl benzene is oxidized in liquid phase at 110~130 ℃, pressure is 0.1~0.49 MPa, isobutyl benzene hydrogen peroxide is formed, and then decomposed in the presence of acid catalyst, the oxidation liquid is concentrated at 20~60 ℃, and then butanone and phenol are produced, and the final product is separated and refined. [2] This method is characterized by light corrosion of process equipment, mild reaction conditions, and favorable to Industrialization:
| Nature of inquiries | Department | Location | Telephone | |
|---|---|---|---|---|
| Sales/distribution | China Methy Ethyl Ketone sales manufacturer | China | +86-19117288062 | service@skychemwin.com |
Chemwin can provide a wide range of bulk hydrocarbons and chemical solvents for industrial customers.
.
Before that, please read the following basic information about doing business with us:
1.security
Safety is our top priority. In addition to providing customers with information about the safe and environmentally friendly use of our products, we are also committed to ensuring that the safety risks of employees and contractors are reduced to a reasonable and feasible minimum. Therefore, we require the customer to ensure that the appropriate unloading and storage safety standards are met before our delivery (please refer to the HSSE appendix in the general terms and conditions of sales below). Our HSSE experts can provide guidance on these standards.
2.Delivery method
Customers can order and deliver products from chemwin, or they can receive products from our manufacturing plant. The available modes of transport include truck, rail or multimodal transport (separate conditions apply).
In the case of customer requirements, we can specify the requirements of barges or tankers and apply special safety/review standards and requirements.
3.Minimum order quantity
If you purchase products from our website, the minimum order quantity is 30 tons.
4.payment
The standard payment method is direct deduction within 30 days from the invoice.
5.Delivery documentation
The following documents are provided with each delivery:
Product Name:Methyl Ethyl Ketone
Molecular format:C4H8O
CAS No.:78-93-3
Product molecular
structure: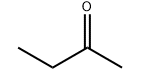
Specification:
|
Unit |
Value |
|
|
Purity |
% |
99.8min |
|
Color |
APHA |
8max |
|
Acid value(as acetate acid) |
% |
0.002max |
|
moisture |
% |
0.03max |
|
Appearance |
- |
Colorless liquid |
Chemical Properties:
Methyl ethyl ketone (MEK) is a colorless liquid with an odor that has been described as moderately sharp, fragrant, peppermint, or acetone like. It soluble in water up to 28% by weight and is miscible with many other organic solvents. The lower explosive limit is 1.4% and the upper explosive limit is 11.4%. Methyl ethyl ketone may be incompatible with strong oxidizers, amines, ammonia, inorganic acids, caustics, isocyanates, and pyridines. When used industrially, methyl ethyl ketone must be handled with caution, as it is a Class lB flammable liquid NIOSH
Application:
Methyl ethyl ketone (2-butanone, ethyl methyl ketone, methyl acetone) is an organic solvent of relatively low toxicity, which is found in many applications. It is used in industrial and commercial products as a solvent for adhesives, paints, and cleaning agents and as a de-waxing solvent. A natural component of some foods, methyl ethyl ketone can be released into the environment by volcanoes and forest fires.It is used in themanufacture of smokeless powder and colorless synthetic resins, as a solvent, and insurface coating. It is also used as a flavoringsubstance in food.
MEK is
used as a solvent for various coating systems, for example, vinyl, adhesives,
nitrocellulose, and acrylic coatings. It is used in paint removers, lacquers,
varnishes, spray paints, sealers, glues, magnetic tapes, printing inks, resins,
rosins, cleaning solutions, and for polymerization. It is found in other
consumer products, for example, household and hobby cements, and wood-filling
products. MEK is used in dewaxing lubricating oils, the degreasing of metals,
in the production of synthetic leathers, transparent paper and aluminum foil,
and as a chemical intermediate and catalyst. It is an extraction solvent in the
processing of foodstuffs and food ingredients. MEK can also be used to
sterilize surgical and dental equipment.
In addition to its manufacture, environmental sources of MEK include exhaust
from jet and internal combustion engines, and industrial activities such as
gasification of coal. It is found in substantial amounts in tobacco smoke. MEK
is produced biologically and has been identified as a product of microbial
metabolism. It has also been found in plants, insect pheromones, and animal
tissues, and MEK is probably a minor product of normal mammalian metabolism. It
is stable under ordinary conditions but can form peroxides on prolonged
storage; these may be explosive.