Min.Order Quantity: Ton
Port: Shanghai
Payment Terms: T/T
Product Name:Calcium carbide
Molecular format:C2Ca
CAS No:75-20-7
Product molecular structure:
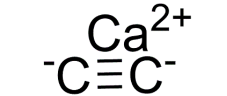
Chemical Properties:
Calcium carbide (molecule formula: CaC2), is a kind of important chemical raw materials produced from the chemical processing of limestone. In 1892, H. Maysan (French) and H. Wilson (United state) simultaneously developed a calcium carbide production approach based on furnace Reduction. The United State had successfully achieved industrial production in 1895. The property of calcium carbide is related to its purity. Its industrial product is mostly the mixture of calcium carbide and calcium oxide, and also contains trace amounts of sulfur, phosphorus, nitrogen and other impurities. With the increasing content of impurities, it color exhibits gray, brown to black. The melting point and electrical conductivity both decrease with the decrease of the purity. The purity of its industrial product is usually 80% with m.p. being 1800~2000 °C. At room temperature, it does not react with air, but it can have oxidation reaction at above 350 ℃, and have reaction with nitrogen at 600~700 ℃ to generate calcium cyanamide. Calcium carbide, when coming across with water or steam, generates acetylene and release a large amount of heating. CaC2 + 2H2O─ → C2H2 + Ca (OH) 2 + 125185.32J, 1kg of pure calcium carbide can produce 366 L of acetylene 366l (15 ℃, 0.1MPa). Thereby, for its storage: calcium carbide should be strictly kept away from water. It is usually packed in a sealed iron container, and sometimes stored in a dry warehouse being filled with nitrogen if necessary.